overview
Pharma with Infinite Paperless Suite
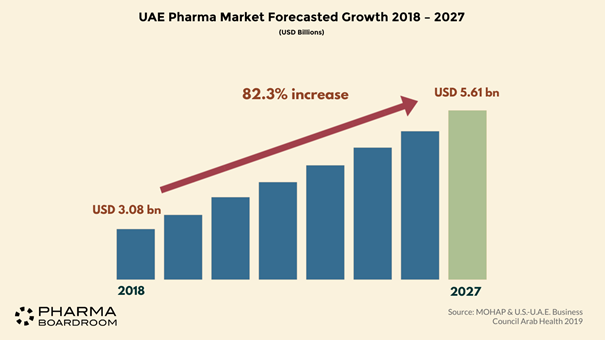
The UAE’s pharma market was predicted to grow to USD four billion in 2020, representing a CAGR of 8.5 percent from 2019. The country sits at the heart of the Middle East which, although only making up two percent of the global pharmaceutical industry, has been growing at a rate of ten percent and is therefore increasingly attracting the eye of international investors.
The UAE pharmaceutical sector appears to be following a path similar to that seen at a regional levels. It is expanding rapidly to meet primarily the evolving needs of a growing population which is expected to reach almost 11.1 million by 2030 according to the world bank projects.
With the full fallout of the global coronavirus pandemic still unknown as of April 2020, the UAE nevertheless seems set to remain a highly sought-after investment destination for the foreseeable future. A raft of multinational pharma companies are basing management for vast regions from the UAE thanks to its stability and openness compared to that of its neighbours, well-developed infrastructure, and attractiveness to top talent.
Looking towards the future, the UAE will need to reconcile importing innovation from overseas with developing technologies at home among other challenges, but the nation’s burgeoning reputation as a gathering force in the global healthcare and life sciences industries seems well justified.
Industry challenges
Pharma challenges
The Pharmaceutical industry is struggling to deal with the challenges that have emerged from supply chain security lapses, counterfeiting and increased regulatory pressure. In fact, most pharmaceutical companies are finding it difficult to ensure the integrity of their products as they move across the value chain i.e. from manufacturers to wholesalers and finally to the end-user (patients). Besides safety concerns, these challenges have adversely impacted the health of the entire industry by reducing profits and impairing brand credibility. Preventing theft and counterfeiting have thus become a key focus for businesses. To remain competitive, pharma companies require a robust and revolutionary technology that can resolve most of the current problems associated with the pharma industry.
The latest regulatory requirements affecting life sciences – including ISO Identification of Medicinal Products (IDMP) and new US Food and Drug Administration (FDA) Standardization of Pharmaceutical Quality/Chemistry Manufacturing and Control (PQ/CMC) data elements and terminologies – are so demanding that it is prompting many companies to completely rethink the way they use product information. And this promises to serve them well, as long as they automate more of what they do with these data assets.

